Afforgen Phase Alpha: A Comprehensive Overview
Afforgen Phase Alpha: A Comprehensive Overview
Related Articles: Afforgen Phase Alpha: A Comprehensive Overview
- 2025 Lexus ES 350: Unveiling A Symphony Of Sophistication And Innovation
- Western Canada Summer Games 2025: A Showcase Of Athletic Excellence And Community Spirit
- The 2025 Audi Q3: A Comprehensive Guide
- Can You Use 2025 Instead Of 2032?
- The 2025 Ford Mustang GTD: A New Era Of Power And Performance
Introduction
With great pleasure, we will explore the intriguing topic related to Afforgen Phase Alpha: A Comprehensive Overview. Let’s weave interesting information and offer fresh perspectives to the readers.
Table of Content
Video about Afforgen Phase Alpha: A Comprehensive Overview
Afforgen Phase Alpha: A Comprehensive Overview

Introduction
Afforgen Phase Alpha is a pivotal Phase 3 clinical trial evaluating the safety and efficacy of affogen, an investigational gene therapy, in patients with hemophilia B. The trial is designed to assess the long-term durability and safety of affogen in a large patient population. This article provides a comprehensive overview of Afforgen Phase Alpha, including its design, endpoints, and potential implications.
Background
Hemophilia B is a rare inherited bleeding disorder caused by a deficiency in clotting factor IX (FIX). Patients with hemophilia B experience recurrent bleeding episodes, which can lead to significant pain, joint damage, and life-threatening complications.
Current treatment options for hemophilia B include prophylactic infusions of FIX replacement therapy. While these treatments can effectively prevent bleeding episodes, they require frequent intravenous infusions and can be associated with side effects. Gene therapy offers a potential cure for hemophilia B by delivering a functional FIX gene to the patient’s cells.
Affogen Gene Therapy
Affogen is an adeno-associated virus (AAV)-based gene therapy that delivers a FIX gene to liver cells. AAVs are naturally occurring viruses that have been modified to be safe and effective for gene delivery. When administered to patients with hemophilia B, affogen is designed to insert the FIX gene into the DNA of liver cells, allowing them to produce functional FIX protein.
Afforgen Phase Alpha Trial
Afforgen Phase Alpha is a multicenter, randomized, double-blind, placebo-controlled trial designed to evaluate the safety and efficacy of affogen in patients with hemophilia B. The trial is enrolling approximately 150 patients aged 12 years and older with severe or moderately severe hemophilia B.
Participants are randomized to receive a single intravenous infusion of either affogen or placebo. The primary endpoint of the trial is the annualized bleeding rate (ABR) over a two-year period. Secondary endpoints include FIX activity levels, quality of life measures, and safety assessments.
Key Features of the Trial
- Long-term follow-up: The trial will follow patients for up to five years to assess the durability of the gene therapy effect.
- Randomized and placebo-controlled design: The trial is designed to minimize bias and ensure that any observed effects are due to affogen.
- Large patient population: The trial is enrolling a large number of patients, which will provide robust data on the safety and efficacy of affogen.
- Comprehensive safety monitoring: The trial includes rigorous safety assessments to ensure the safety of affogen.
Potential Implications
If Afforgen Phase Alpha is successful, it could have significant implications for the treatment of hemophilia B. A safe and effective gene therapy could provide a long-term cure for patients, eliminating the need for frequent prophylactic infusions and reducing the risk of bleeding episodes.
Furthermore, the success of Afforgen Phase Alpha could pave the way for gene therapies for other bleeding disorders and genetic diseases.
Timeline
Afforgen Phase Alpha is expected to be completed in March 2025. Top-line results from the trial are anticipated in mid-2025.
Conclusion
Afforgen Phase Alpha is a pivotal clinical trial that is evaluating the safety and efficacy of affogen, an investigational gene therapy for hemophilia B. The trial is designed to assess the long-term durability and safety of affogen in a large patient population. If successful, Afforgen Phase Alpha could have significant implications for the treatment of hemophilia B and other genetic diseases.
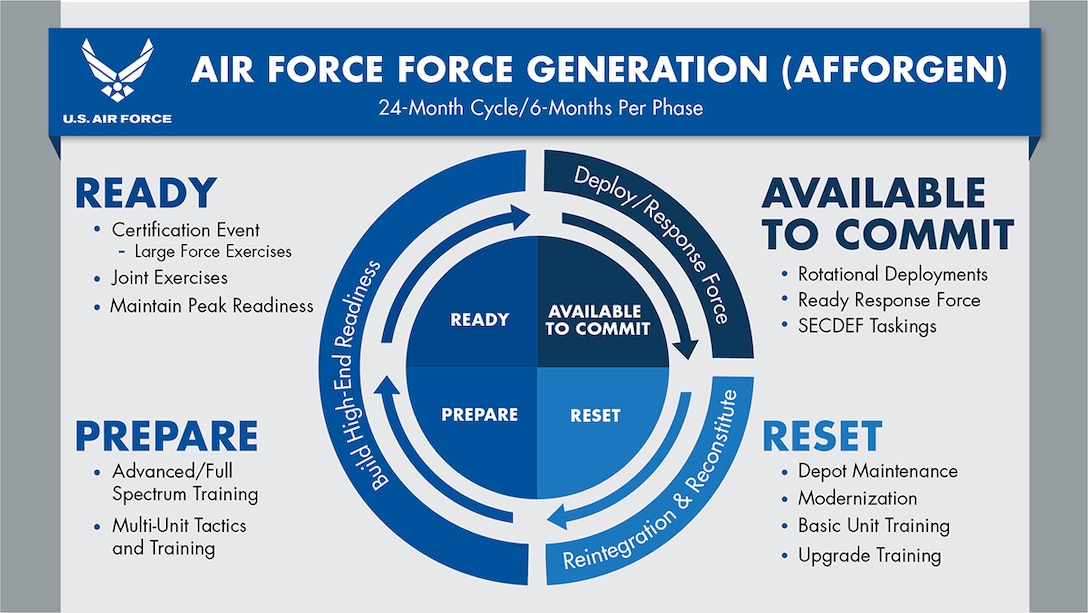


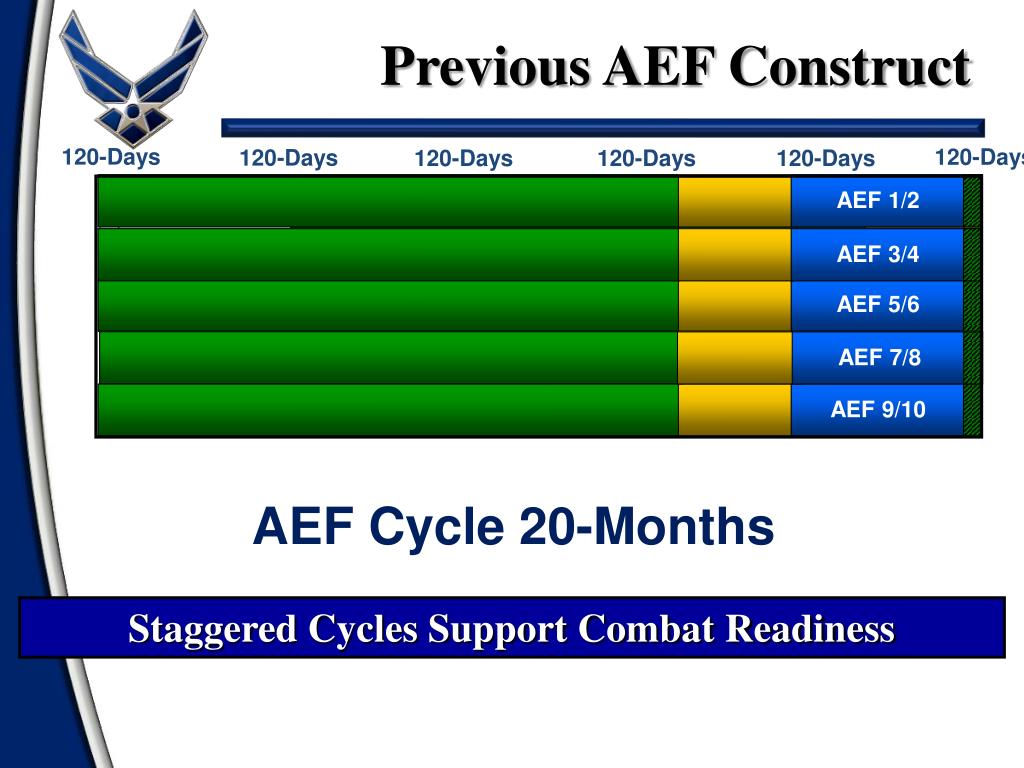
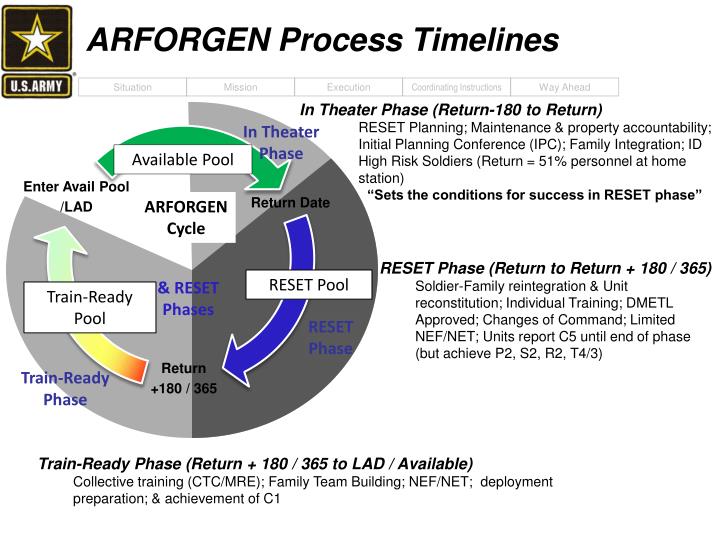
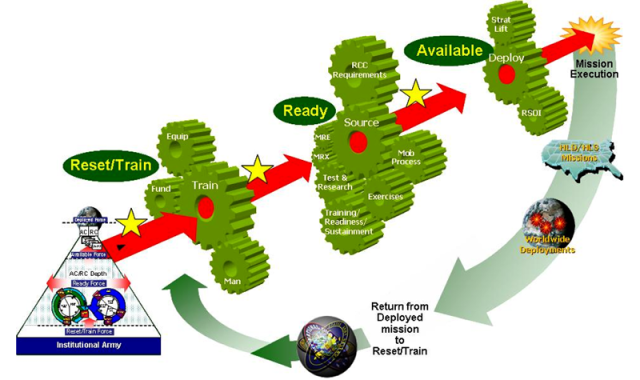

Closure
Thus, we hope this article has provided valuable insights into Afforgen Phase Alpha: A Comprehensive Overview. We appreciate your attention to our article. See you in our next article!
